Many anti-snoring products make promises. ONIRIS® brings you results.
The ONIRIS® orthosis is
the only
self-fitting anti-snoring device whose effectiveness has been evaluated and proven in hospitals and clinics by sleep specialists, ENT specialists and dentists in France (1,2).
The ONIRIS® orthosis is also the only self-adapting thermoformable orthosis whose effectiveness is no less than that of custom-made orthoses, which cost 10 times more.
These reliable clinical studies have been carried out in practices, clinics and public institutions such as AP-HP (Assistance Publique-Hôpitaux de Paris), Grenoble University Hospital and Montpellier University Hospital. They have been controlled by an independent laboratory using protocols validated by the ANSM, the French Data Protection Authority and the CNIL, as well as a scientific committee made up of experts in the fields of Sleep, Odontology and Biostatistics.
These rigorous studies have also shown that the ONIRIS orthosis offers excellent long-term tolerance:
- 93% of users have good compliance.
- The Oniris orthosis is worn 89% of sleep time. This means wearing it all night long 6 days a week
- 73.3% of users report no discomfort at all, and any discomfort experienced is considered minor or negligible.
- 97% have no significant dental or joint impact.
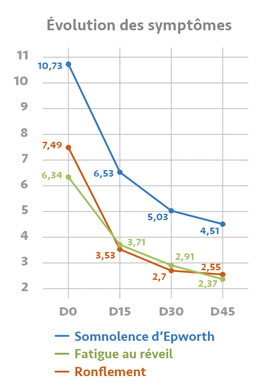
ONIRIS® effectiveness in figures :
(1) Marty, M., et al. (2015), Snoring and Obstructive Sleep Apnea: Objective Efficacy and Impact of a Chairside Fabricated Mandibular Advancement Device. American Journal of Prosthodontics. doi:10.1111/jopr.12401. A prospective, multicenter, open-label, uncontrolled study conducted at the Assistance Publique-Hôpitaux de Paris, with the primary objective of evaluating the efficacy of the ONIRIS thermoformed mandibular advancement orthosis in 41 patients with snoring and severe sleep apnea syndrome who refused or discontinued CPAP. The follow-up period was 45 to 60 days, monitored by an independent laboratory using a protocol validated by the AFSSAPS, the Comité de Protection des Personnes SUD Med IV, the CCTIRS and the CNIL.
(2) Pepin J. L. et al. Effect of custom made vs thermoplastic heat molded mandibular advancement devices (MADs) for Obstructive Sleep Apnea (OSA): A randomized non-inferiority trial. Abstract supplement. European Respiratory Journal. September 2017. Open-label randomized controlled study conducted in university hospitals, clinics and medical practices (Bordeaux, Grenoble, Perpignan, Paris, Versailles, Montpellier, Bézier, Pavillons-sous-Bois) with the primary objective of comparing the efficacy of the ONIRIS thermoformed mandibular advancement orthosis versus the custom-made TALI orthosis (validated by the French National Authority for Health) in 204 patients with snoring and sleep apnea syndrome greater than 15/hour, refusing or discontinuing CPAP. The follow-up period was 360 to 390 days, and the results were checked by an independent laboratory using a protocol validated by a scientific committee, the ANSM, a Comité de Protection des Personnes and the CNIL.
